PROJECT

Strategy and associated methods:
BUR-EB is a person-centred proposal based on mixed-methods research, with two main components:
A) Cross-sectional study: Data on the socio-economic burden will be collected via an anonymous survey in collaboration with clinicians and patient organizations (DEBRA). The economic burden will comprise healthcare and informal care costs, financial burden for families and productivity losses. Quality of life and family burden will be measured by validated scales.
B) Qualitative study: Patient Journey Maps with trajectories of care per each EB type and 2-4 capacity building informational materials for self-management will be developed by a co-creation process using online focus groups and workshops followed by asynchronous participation until optimized versions of the deliverables are reached. BUR-EB is backed-up by a balanced multidisciplinary group of internationally recognized clinical dermatologists, health economists, qualitative research experts, biostatisticians and patient representatives.
Expected results and impact:
BUR-EB offers a unique opportunity to evaluate changes in the socio-economic burden in the last decade, which could provide significant insights into the practical implementation and long-term results of the EU Council Recommendation of 2009, which has been guiding both EU and MS rare disease policy over the last decade, as well as the impact of country-specific policies, strategies and interventions. Tools developed in BUR-EB can be useful for the foreseen studies of novel therapeutic options or service design, as well as the assessment of new policy interventions.
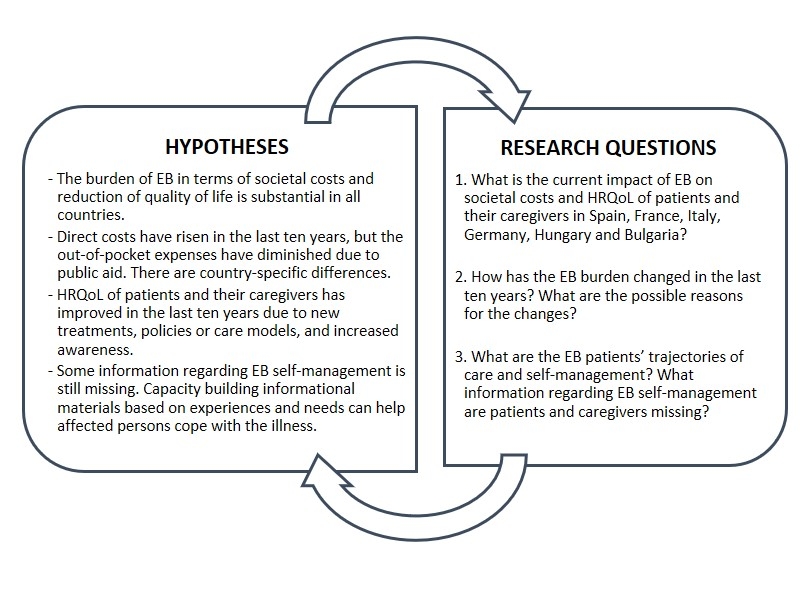
Work packages:
The research strategy consists of five phases, corresponding to five work packages (WP):
Leader: IIER (Spain), co-leader: FIISC
a) Methods revision — Key partners: HECON; Collaboration: BAPES.
Update of the methods and tools developed in the BURQOL-RD project in 2011-2013 to measure the socio-economic burden of rare diseases. Improvements of the methods will be proposed.
b) Situational analysis — Key partners: DEBRA International, OPBG, UKF, NEMH; Collaboration: DEBRA associations in each country.
Analysis of the situation and the development in the last decade regarding the management of EB, possible new treatments or care models, number and distribution of reference centres and services, and patients’ access to them will be carried out in each participating country. The situation during the COVID-19 pandemic will be analysed separately. The results will serve as inputs for WP2, WP4 and WP5.
Leader: NEMH (France)
The outcome of WP1 will allow a questionnaire to be developed to gather data on EB burden in WP3 and to analyse them in WP4.
a) EB resource utilization – Key partners: FIISC, HECON, DEBRA International, BAPES
Resource use related to EB will be identified with the help of clinical experts (OPBG, UKF, NEMH) and PAO (DEBRA Int.). Questions will be designed in order to be able to gather direct costs and productivity losses, but also information on out-of-pocket expenses and time utilization for informal care. Aspects outside the healthcare sector will be considered (e.g., social, employment or educational) where disease-related costs can be incurred.
b) Patient-reported outcome measures – Key partners: OPBG, UKF, IIER, DEBRA International
Patients’ HRQoL measured by the generic EQ-5D-5L/Y tool and a dermatology-specific tool, such as the IDLQI; caregivers’ HRQoL measured by EQ-5D-5L and the caregivers’ and family burden specific (Zarit scale/EB-BoD); assessment of patients’ and caregivers’ well-being (WHO-5/happiness scale/ICECAP), access to and satisfaction with social and healthcare services (PREM), covered and uncovered needs, and others. Specific information about healthcare during the COVID-19 pandemic will be gathered: i) consequences of the pandemic on the level of care (missed visits, alternative care models, satisfaction) ii) COVID-19 vaccination completion/modalities in EB patients in 6 countries.
c) Questionnaire preparation – Key partners: OPBG, UKF, FIISC, HECON, IIER, BAPES; Collaboration: DEBRA associations in each country
The questionnaire will be translated into the languages of the participating countries and it will undergo cross-cultural adaptation in each country. It will be pilot-tested on a small group of EB patients/caregivers in each country. These country-specific questionnaires will then be converted into an online format, using an on-line survey tool.
Leader: DEBRA International
Survey – Key partners: FIISC, OPBG, UKF, NEMH, HECON; Collaboration: DEBRA associations in each country
A cross-sectional collection of data from EB patients and their caregivers will be carried out, following Ethical Committee approval in each country. Patients and/or their caregivers will be contacted via PAO (DEBRA associations in each country) and will be invited to complete an anonymous online questionnaire. Those participants without Internet access will be offered the possibility of completing it during medical visits. All participants will be informed about the study objectives and data confidentiality, and they will be asked to indicate their understanding of the study conditions and provide informed consent to participate.
Leader: HECON (Hungary)
a) Analysis of BURQOL-RD data (collected in 2011-13) – Key partners: FIISC, IIER
The revised methods of HRQoL measurement (new value sets developed by PECUNIA) and cost valuation (PECUNIA costing templates to calculate unit costs) will be first applied to EB data collected in BURQOL-RD in all involved countries. These results will serve as the baseline for a before-after study.
b) Analysis of present data (collected in 2022-23) – Key partners: FIISC, OPBG, UKF, NEMH, IIER, BAPES
Data will be analysed per country. Costs will be divided into 4 categories: direct health care costs (drugs, medical visits, examinations, dressings, wheelchairs, other devices); direct non-health care formal costs (professional caregivers, social services, travel and accommodation, house adaptation); direct non-health care informal costs (unpaid carers); and productivity losses (patient’s and carer’s temporary and permanent sick leave or early retirement). HRQoL and well-being results will be analysed according to each scale. The same value sets and costing methods as in point a) will be used.
c) Analysis of changes – Key partners: same as in a) and b)
Comparison of the burden of EB today and 10 years ago will be performed, in order to reflect the possible changes due to the introduction of new treatments, policies or care models. Possible drivers of these changes will be analysed, as well as the impact of COVID-19.
Leader: FIISC (Spain)
a) Patient’s Journey Maps and prioritization of capacity building needs – Key partners: DEBRA International. Collaboration: DEBRA associations in each country
Patient’s Journey Maps (=schemes to reflect the trajectory of care) per EB type will be developed through an iterative process: online focus groups with clinicians and online workshops with EB patients and their caregivers, divided into subgroups by EB type. The experiences and needs of EB patients regarding self-management (patient empowerment, shared decision making, capacity building) will be collected and prioritized during the workshops. A draft of Patient Journey Maps will be developed among participants, using asynchronous online participation (questionnaires, social networks or emails), until consensus on design and content is reached.
b) Capacity building informational materials for self-management — Key partners: DEBRA International. Collaboration: DEBRA associations in each country
Informational materials (between 2 and 4) will be developed for the prioritized capacity building needs. The exact number of materials, extension and format (e.g., online printable brochures or video recordings with subtitles) will be decided during the co-creation process, using questionnaires, social networks or emails. EB patients, caregivers and clinicians will participate in online workshops to develop each material that will be based on their experiences, clinical guidelines and other relevant evidence-based products. The materials will be developed in English and translated to the languages of the participating countries.
Leader: FIISC (Spain)
The management of the project will be organized according to the following scheme:
a) The Project Management Team (PMT) is in charge of the negotiation and immediate activation of the project after the signing of the contracts. The PMT will also:
– maintain direct contacts with the Joint Call Secretariat and communications with the Partners;
– organize meetings in a presential or virtual format (kick-off, mid-term and final);
– check the progress of the work and deliver the 18-month periodic and final reports of the project;
– look after the formal closing of the work.
b) The Steering Committee constituted by the PMT, PIs of each research partner and PAO representative. WP leaders represent the structure for ensuring the correct progress of the project. Ad hoc meetings will be organized if required.
c) The Advisory Board will be composed of international experts representing the major stakeholders involved in the clinical management of patients with EB and in health economics. It will give advice on major topics and will evaluate the project‘s progress and the sustainability plan.
d) The General Assembly will be constituted by the PMT, Partners, Collaborators and PAOs. It will be in charge of all strategic decisions.
Leader: OPBG (Italy)
The communication and dissemination of the results achieved throughout the project will strengthen the overall impact of project activities. The study results will be published after the final analysis in the form of scientific articles in peer-reviewed journals or as presentations at national and international conferences.
The dissemination strategy will also be supported by all partners and PAOs, who will present the results to patients and their families through their respective websites and meetings.
Dissemination to the general public to raise awareness of care problems and socio-economic burden of EB will be in the form of press releases in each country and social media.